Low Dose Naltrexone: Is it Really Worth the Hype?
Nov 6, 2023, by Priyanka Singla, MD, Yasmin Sritapan, DO, and Bhavana Yalamuru, MD
Introduction
Chronic pain affects over 50 million people in the United States,1 presenting significant challenges in treatment. As we grapple with the current opioid epidemic, there has been a renewed focus on discovering effective non-opioid alternatives to treat chronic pain with minimal adverse effects. Naltrexone is an FDA approved opioid antagonist for treatment of opioid use disorder and alcohol use disorder.2 There is emerging evidence for use of a low dose form of this medication to treat chronic pain conditions. This review will discuss the pharmacology and current evidence for use of Low Dose Naltrexone (LDN) for treating chronic pain.
Pharmacology: Mechanism of Action
Neuropathic pain occurs due to nerve damage, which results in activation of glial cells and subsequent release of proinflammatory mediators leading to peripheral and central sensitization, contributing to the chronic pain experience.3,4 LDN offers a potent therapeutic strategy for mitigating this chronic inflammation and central sensitization via its anti-inflammatory and immunomodulating properties.5-7
Naltrexone is a mu-opioid receptor antagonist.5,8-10 However, it has multiple dose-dependent pharmacological dynamics with different respective effects8,9 and can be a versatile tool to treat and manage chronic pain. At low doses, LDN reduces glial inflammatory response by antagonism of Toll-like receptor 4 in both the central and peripheral nervous system, thereby reversing neuropathic pain.8-11 Additionally, LDN competitively inhibits binding of Opioid Growth Factor to Opioid Growth Factor Receptor for a short duration and therefore results in inhibition of cellular proliferation.5,8 This may have a potential role in cancer treatment as well.5,8
An Important Tool in the Pain Physician’s Armamentarium: Indications and Evidence
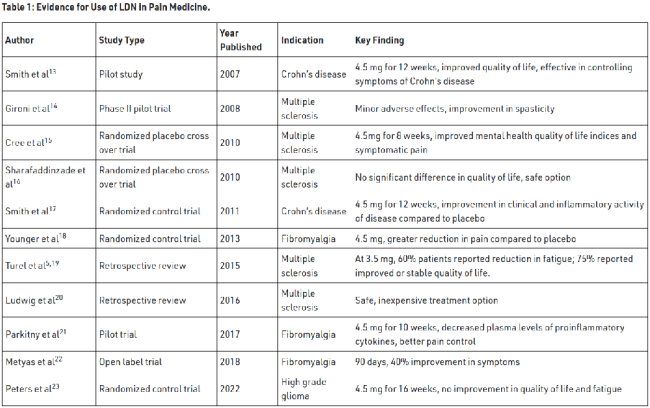
LDN has been utilized in the management of a number of chronic inflammatory and central pain sensitization conditions.5 These include fibromyalgia, Crohn’s disease, multiple sclerosis (MS), complex regional pain syndrome (CRPS), Hailey-Hailey disease, and cancer.7,8 In a recent case series, there was a pronounced therapeutic effect of LDN in patients diagnosed with neuropathic pain in comparison to those presenting with arthritic conditions.12 This has been particularly evident in patients afflicted with painful neuropathic conditions, such as diabetic peripheral neuropathy and CRPS, wherein LDN implementation has yielded successful results.5,12,24,25 However, the broadest research has been conducted in patients with MS and Crohn’s disease. These studies have demonstrated the tangible benefits of LDN therapy in managing and mitigating the often-debilitating effects of these conditions (Table 1).
Concerns with LDN Therapy
In a retrospective review of 215 patients diagnosed with MS, LDN was deemed safe with negligible adverse effects.5,19 Multiple other studies tout the safety profile of LDN.15,16 The most common side effects reported with LDN therapy are sleep disturbances, vivid dreams, fatigue, and headaches.13,17,18 Authors reported resolution of vivid dreams and headaches by decreasing the dose to 3 mg or altering the time of administration from night to day time.12,18
Unfortunately, at the time of writing this review, several insurance carriers in the United States do not pay for or cover the cost of LDN prescriptions.12,19 LDN is specially prepared, which limits its availability to select compounding pharmacies.12 In a retrospective review of cases, McKenzie-Brown et al noted about 1-3 months of lag period of symptom relief in patients who responded after the initiation of therapy.12
Perioperative Concerns for Patients on LDN
When managing patients on LDN, recommendations from the Low Dose Naltrexone Research Trust26,27 suggest stopping LDN 2 days prior to the procedure and restarting 2 days after the patient has stopped taking opioids completely. This recommendation is grounded in the understanding that opioids should be individualized with cautious monitoring, as there can be significant variation in response to narcotics given baseline upregulation of opioid receptors. Most hospital pharmacies don’t carry LDN on formulary and have varying policies on patients bringing their own home medications. Given these circumstances, it may be considered more practical to advise patients to discontinue LDN during the perioperative period of their hospitalization.
How We Do It: Our Practice at the University of Virginia Medical Center
In our practice, we prescribe LDN for patients with fibromyalgia and neuropathic pain conditions such as Complex Regional Pain Syndrome, which are refractory to first- and second-line medications commonly used to treat neuropathic pain and Ehlers Danlos Syndrome. Since patients must pay out of pocket, we reserve it as a last resort when all indicated medications and therapy options have been exhausted.
We initially started patients on LDN at 1.5 mg for a month with subsequent titration to 3 mg in the following month. The objective of this regimen was to attain the target dose of 4.5 mg once daily, which was typically achieved after 2 months of initiating the therapy. We have seen a very low incidence of adverse effects within this protocol and have now changed our practice to start patients at 4.5 mg daily dosage, thereby eliminating the need for a titration period. We follow up with patients in 3 months after starting the medication and thereafter every 6-12 months.
References
Go to article for references.







